Budoprutug and CD19
Budoprutug is an anti-CD19 mAb designed to result in potent and durable B cell depletion, with a long dosing interval and the potential for both intravenous (IV) and subcutaneous (SC) administration.
CD19 is an attractive target relevant to multiple B cell-mediated diseases, as it is expressed across the B cell lineage, including plasmablasts. This broad CD19 expression profile may enable comprehensive, rapid, and durable depletion of pathogenic B cells while preserving protective antibody responses mediated by long-lived plasma cells. As such, targeting CD19 represents a differentiated approach to addressing immune-mediated disease.
Budoprutug is a highly potent, Fc-enhanced anti-CD19 mAb
Budoprutug is designed for optimal biological activity and has the potential for both intravenous (IV) and subcutaneous (SC) delivery. The ability to formulate as both IV and SC provides optionality in development and may be a differentiating feature of budoprutug relative to other anti-CD19 mAbs.
A completed budoprutug Phase 1b study in pMN demonstrated proof of concept and a favorable safety profile
- Rapid and complete circulating B cell depletion observed in 100% (5/5) at doses of 100-200 mg (IV administration)
- All evaluable patients (3/3) achieved serologic remission, as measured by anti-PLA2R antibody negativity
- All patients (5/5) achieved complete or partial remission by week 48, as measured by Urine Protein Creatine Ratio (UPCR)
- Budoprutug was generally well tolerated at doses of up to 200 mg, supporting exploration of higher doses in future studies
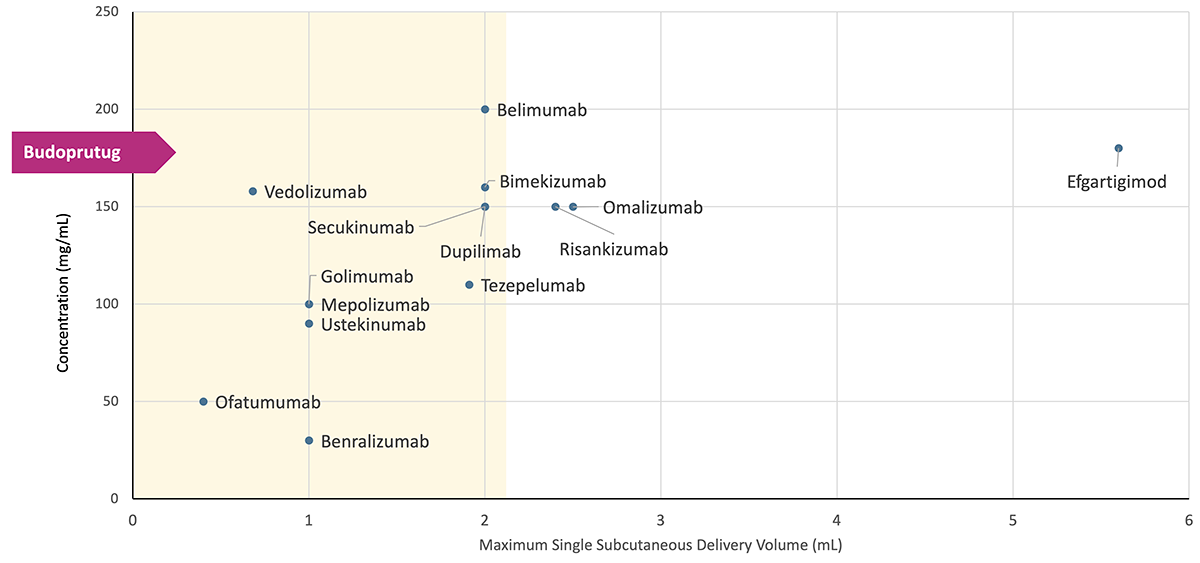
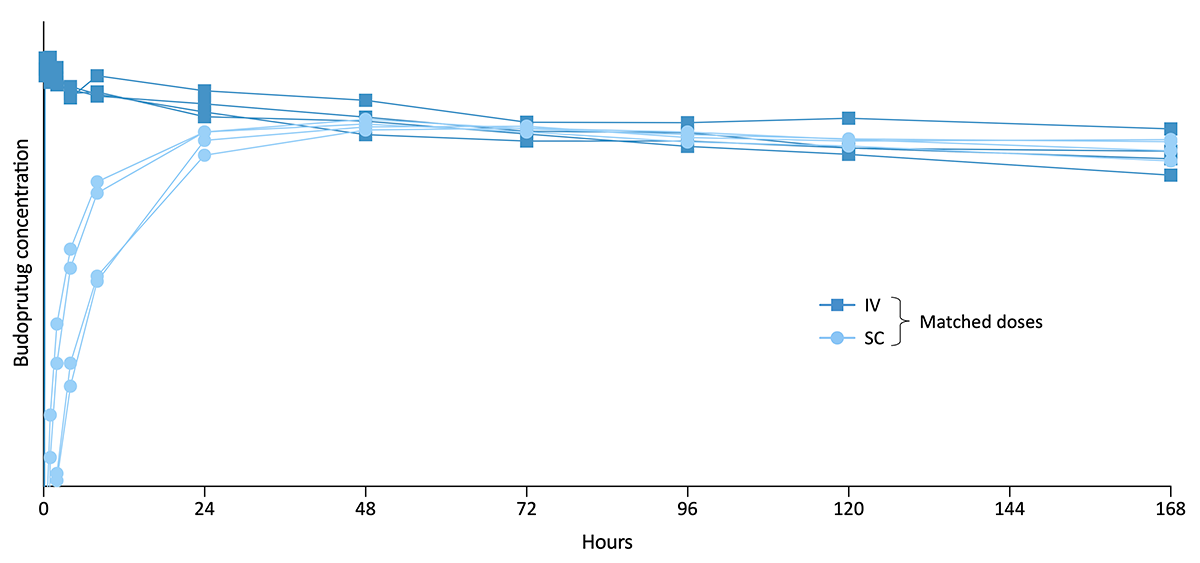
Budoprutug subcutaneous (SC) formulation
SC administration has the potential to enable at-home dosing, reduce reliance on healthcare facilities, improve patient convenience, and broaden the range of patients and indications we can serve. The SC formulation of budoprutug is designed to offer greater flexibility for patients and providers.
Data from a study in nonhuman primates supports the viability of our SC budoprutug formulation. In this study, budoprutug demonstrated high bioavailability (>90%) and favorable tolerability, with no observed drug-related safety findings. Together, budoprutug’s high concentration, low viscosity, and high bioavailability provide the potential for a high concentration, single SC injection.
A Phase 1 study assessing the safety and tolerability of the SC formulation of budoprutug in healthy volunteers is currently underway.
Our budoprutug development strategy
We are developing budoprutug across multiple disease categories, pursuing lead indications with high unmet need, clear B cell-driven pathology and large therapeutic and commercial opportunities. The results from ongoing clinical studies will inform the design of later-stage trials and potential additional expansion into new indications.
IgG4-mediated Conditions
Diseases with clear pathophysiology that support targeting of CD19-expressing B cells with demonstrated clinical proof of concept
Single Organ, Orphan Diseases
Diseases for which B cell depletion has demonstrated clinical proof of concept
Complex Systemic Disorders
Multi-organ diseases with a more heterogeneous patient population; early clinical validation for CD19 via other modalities
Clinical Trials
We have several clinical studies ongoing for budoprutug, evaluating multiple disease areas and exploring different routes of administration.